The standardization of HbA1c testing is crucial for accurate diagnosis
With the development of the global economy, changes in lifestyle, environmental shifts, an aging society, and advancements in healthcare services, the incidence and prevalence of non-communicable chronic diseases (NCDs) have been increasing, resulting in a heavy burden of medical expenses on individuals, families, and society. Diabetes, a chronic condition characterized by high blood sugar levels, can cause long-term damage, functional impairments, and multiple organ failures. It is currently the third leading cause of death, following cardiovascular disease and cancer.
According to the International Diabetes Federation (IDF) report in 2013, there were 382 million diabetes patients worldwide (8.3% of the adult population), with 175 million undiagnosed cases (46%) and 5.1 million deaths. The cost of diabetes treatment reached $548 billion (11% of global healthcare expenditure), and it is projected that the number of diabetes patients worldwide will reach 592 million (11% of the adult population) by 2035. In China, the Chinese Diabetes Society (CDS) reported that there were 98.4 million diabetes patients in 2013 (aged 20-79), with 53.2 million undiagnosed cases and 1.27 million deaths. The average medical expenditure for diabetes treatment per person was $333. China has the highest number of diabetes patients, followed by India (65.1 million) and the United States (24.4 million). Diabetes has become a global public health issue.
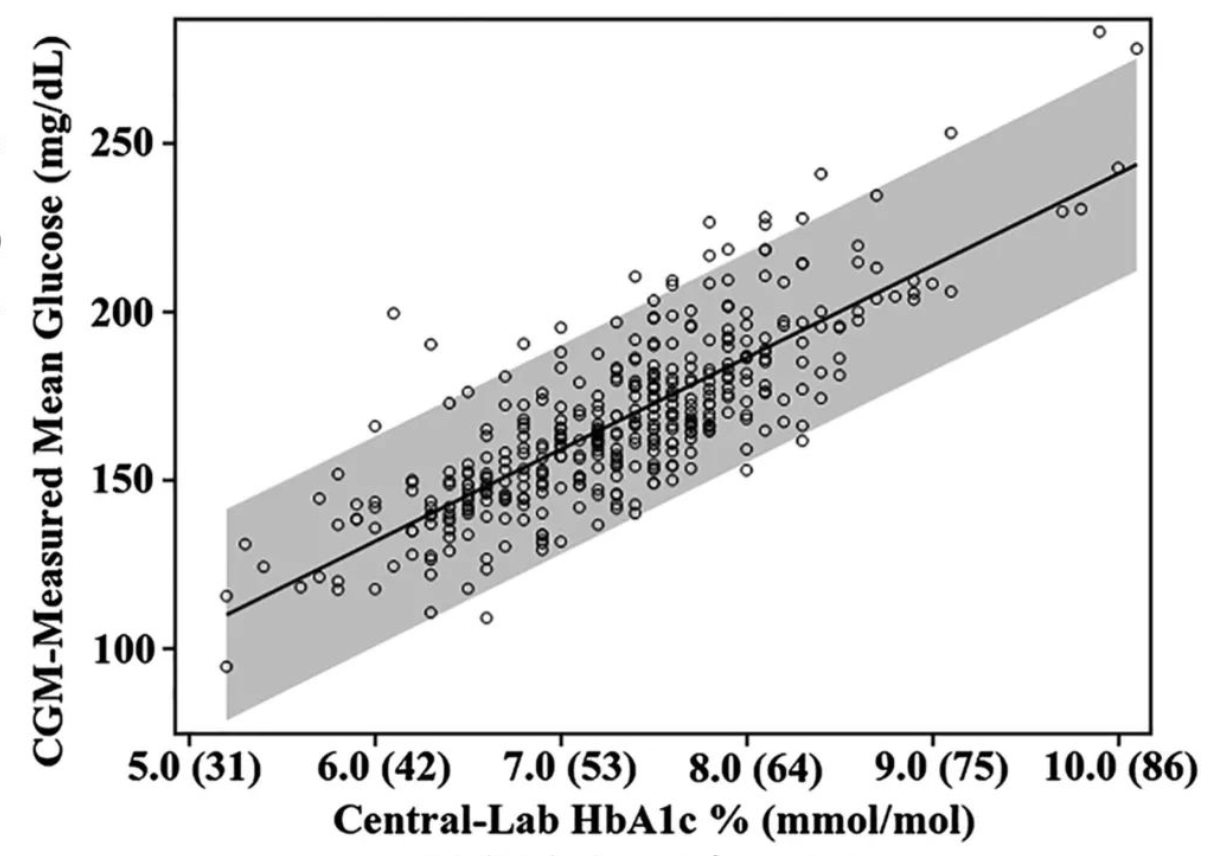
The diagnosis of diabetes traditionally uses fasting plasma glucose (FPG) and oral glucose tolerance test (OGTT). However, these methods have limitations and can be influenced by various factors. The introduction of glycated hemoglobin (HbA1c) as a diagnostic tool has gained popularity. HbA1c reflects a person's average blood sugar levels over the past 2-3 months and has advantages over traditional blood glucose testing in terms of stability and convenience. In 2010, the American Diabetes Association (ADA) officially included HbA1c as a diagnostic method for diabetes, with a cutoff point of ≥6.5%.
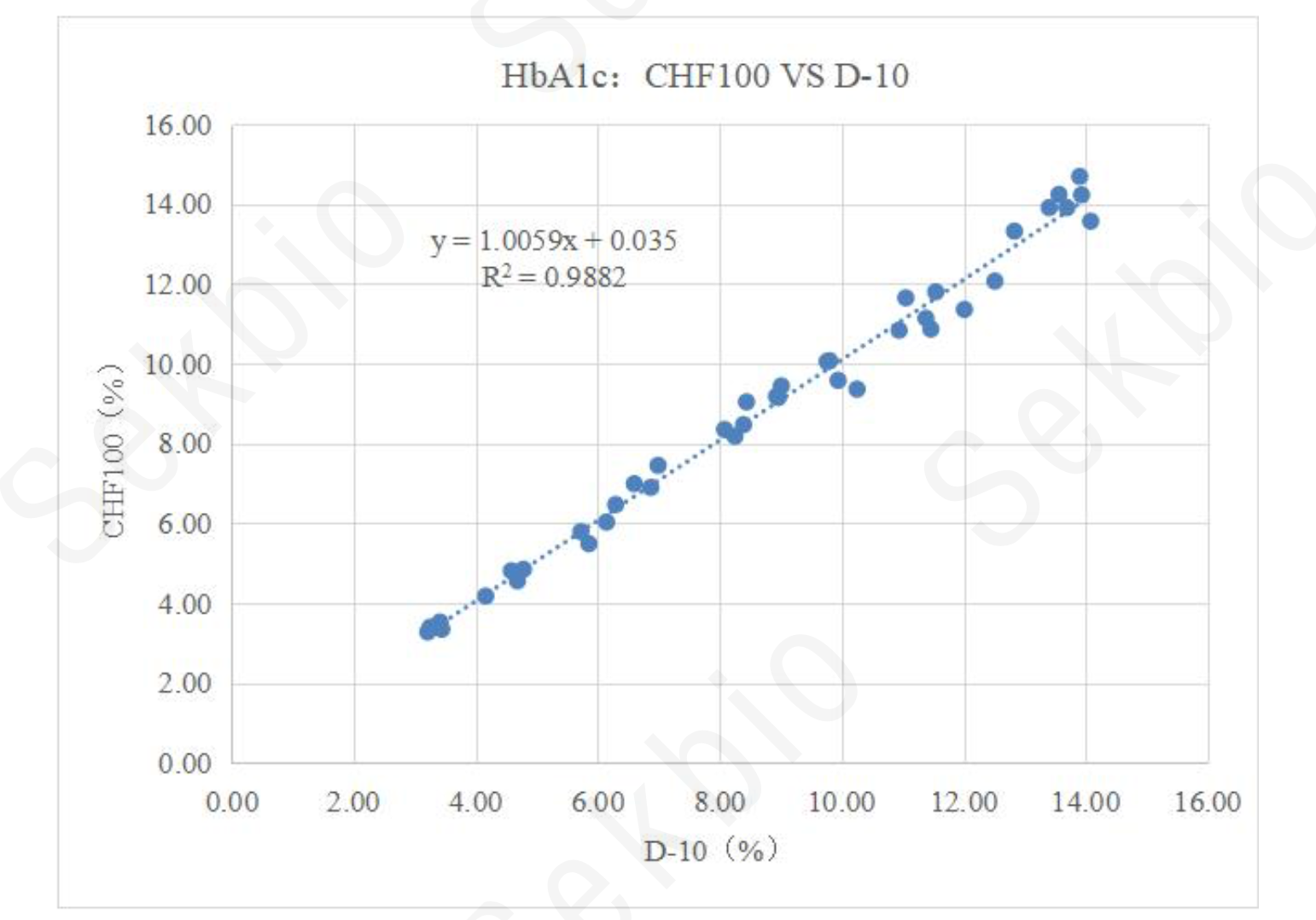
HbA1c measurement involves various laboratory methods, including chromatography, electrophoresis, immunoassay, enzymatic methods, and rapid tests. Different methods can yield significant differences in results. To ensure comparability and traceability, international standardization efforts have been made. The National Glycohemoglobin Standardization Program (NGSP) and the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) have established reference systems and networks of reference laboratories. Currently, over 150 clinical laboratories have achieved NGSP certification, and IFCC and NGSP are working together on harmonization.
In China, efforts to standardize HbA1c testing and improve quality are ongoing. Three laboratories have been accepted by the IFCC as level 1 reference laboratories, and the National Center for Clinical Laboratory and the Beijing Center for Clinical Laboratory have joined the network. These laboratories have developed quality control measures and proficiency testing programs to enhance testing quality. National and industry standards are being revised to ensure product quality and laboratory practices.
Although HbA1c has not been included as a diagnostic criterion in Chinese guidelines, progress in standardization has been made, and the overall quality of HbA1c testing has improved. Further efforts are needed to promote HbA1c testing and achieve full standardization in China.
In conclusion, the standardization of HbA1c testing is crucial for accurate diagnosis, effective management, and better healthcare outcomes for diabetes patients. International collaboration and ongoing efforts in China are paving the way for the use of HbA1c as a standardized and reliable test in clinical practice.