Raw Materials for COVID-19 Neutralizing Antibody Detection
SEKBIO optimized a batch of RBD antigens and ACE2 protein with high sensitivity, specificity, and low inter-batch difference to help evaluate the neutralizing antibody titer of the COVID-19 vaccine, accelerating the progress of neutralizing antibody titer detection and data collection. The development of neutralizing antibody detection kits is also supported.
Here comesCOVID-19 neutralizing antibody detection. What is it, and how do we find it?
Now, follow my steps to know something about COVID-19, vaccines, and neutralizing antibodies. You will get an accurate answer.
01 The appearance of the 'enemy.'
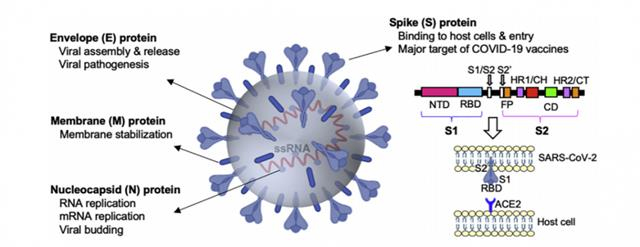
The public knows it is the new type of coronavirus that caused the COVID-19 pneumonia epidemic, which was named 'SARS-CoV-2' by the International VirusClassification Committee. This virus was very similar to the SARS virus in 2003. The genome of the COVID-19 virus mainly encodes four structural proteins, spike (S) protein, envelope (E) protein, membrane (M) protein, and nucleocapsid (N) protein (Figure 1).
02 Intrusion mechanism
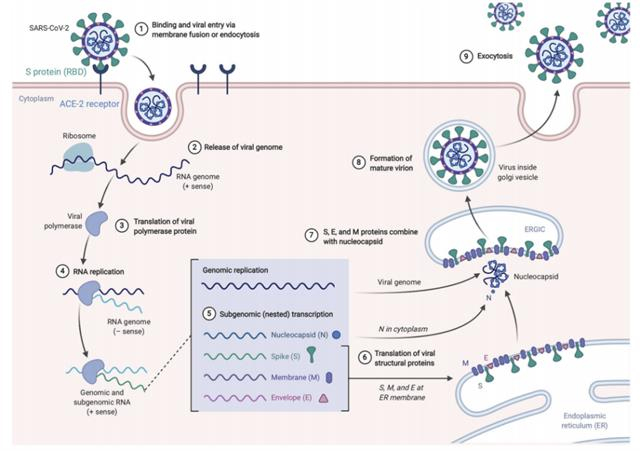
Studies have shown that COVID-19 virus infection in the human body is S protein binding to the receptor angiotensin-converting enzyme 2 (ACE2) on oral epithelial cells, alveolar type II epithelial cells, and other respiratory tracts to enter cells. And begin to replicate and proliferate (Figure 2).
Therefore, to prevent the virus from replicating, it's vital to stop it from combining with ACE2 and entering the cell. What is going to prevent the virus from binding to ACE2? The answer is neutralizing antibodies.
03 Neutralizing antibodies and antigen neutralizing epitopes
The neutralizing antibody is a protective antibody produced by the immune system to recognize viruses, bacteria, toxins, etc. It prevents them from binding to host cells and exerts a protective effect.
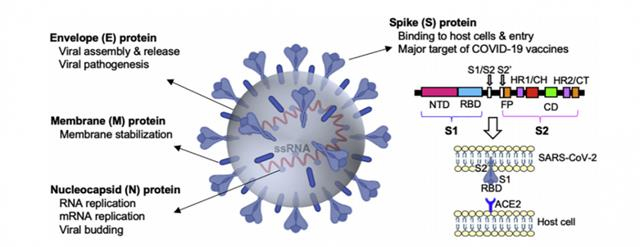
Let's look back at Figure 1. There is a receptor-binding domain on the S protein of the COVID-19 virus, called the receptor-binding domain (RBD), which directly binds to ACE2 on human cells. Studies have confirmed that S-RBD is the primary epitope of neutralizing antibodies. The specific antibodies produced against S-RBD can block the binding of the COVID-19 virus to ACE2 so that the virus cannot invade host cells and play a role in 'neutralizing' the virus, so name it, neutralizing antibody.
04 The role of vaccines
What is Immunity? It refers to the ability of the body to resist infection through infection or artificial vaccination, and vaccines simulate the invasion of pathogens and cause an immune response after entering the human body, prompting the body's immune system to produce protective antibodies.
The inactivated virus vaccine uses the whole virus particle as the immunogen. In addition to its S protein can cause the human immune response to produce protective neutralizing antibodies. A large amount of N protein it contains may also cause the immune response to produce antibodies. Still, the antibody produced by the N protein cannot prevent the COVID-19 virus from binding to ACE2, so it has no protective effect and cannot be called a neutralizing antibody. Cause the recognized domains of the E protein and M protein are small, it is generally considered that they are not the main targets of the immune response.
05 Vaccine Evaluation
The 'Guiding Principles for Clinical Evaluation of COVID-19 virus Preventive Vaccines' pointed out that the primary endpoint of effectiveness evaluation should prevent the onset of COVID-19 to ensure the widely used COVID-19 virus vaccines on the market can produce the expected effects. And the protective effect of the target population is best to achieve more than 70%. After the vaccine is marketed, it is necessary to continue to observe the safety and clinical protection effect in the case of large-scale vaccination and continue to research the durability of protection.
The traditional method of evaluating vaccines is to verify the efficacy of neutralizing antibodies through neutralization experiments using live viruses. The operation is complicated, time-consuming, and high-risk, which significantly inconveniences the large-scale evaluation of the effect of population vaccination. Therefore, it's significant to establish a method for rapid detection of neutralizing antibodies.
06 Neutralizing antibody rapid detection
Based on the characteristics of the combination of neutralizing antibodies and COVID-19 virus and the mechanism of virus infection, there are two strategies for rapid detection of neutralizing antibodies:
One is the double antigen sandwich method that specifically binds neutralizing antibodies by S-RBD. This method only uses S-RBD as the antigen to detect total neutralizing antibodies, including IgG, IgM, and IgA against S-RBD neutralizing epitopes with higher sensitivity and specificity than catching IgG or IgM alone.
The other is the competition strategy, which uses genetic engineering technology to express the S-RBD protein and ACE2 protein to simulate the binding of the virus to the receptor on the natural cell. The neutralizing antibody will inhibit the combination of two proteins.
To help evaluate the neutralizing antibody titer of the COVID-19 vaccine, accelerate the progress of neutralizing antibody titer detection and data collection, SEKBIO optimized a batch of RBD antigens and ACE2 protein with high sensitivity, specificity, and low inter-batch difference. The development of neutralizing antibody detection kits is also supported.
Neutralizing Antibody Detection Materials Ordering Information
Item No. | Product Name | Purity | Concentration |
ZLP811A2 | ACE2 Coated Antigen | >90% | 0.5-1.0 mg/ml |
ZLP811A3 | ACE2 Coated Antigen | >90% | 0.5-1.0 mg/ml |
ZLP811A1 | ACE2 Coated Antigen | >90% | 0.5-1.0 mg/ml |
ZLP81181 | RBD Labeled Antigen | >90% | 2-5 μg/ml |
ZLP81160 | RBD Labeled Antigen | >90% | 2-5 μg/ml |
ZLP811109 | RBD Labeled Antigen | >90% | 2-5 μg/ml |
ZLP811106 | RBD Labeled Antigen | >90% | 2-5 μg/ml |
ZLA81105H | Humanized RBD Positive Quality Control Material | >95% | 0.1-25 μg/ml |
【References】
[1]Jee YoungChung, Melissa N. Thone, Young Jik Kwon. COVID-19 vaccines: The status and perspectives in delivery points of view. Advanced Drug Delivery Reviews, 170(2021) 1–25.
[2]Wanbo Tai1, Lei He, et al.Characterization of the receptor-binding domain (RBD) of 2019novel coronavirus: implication for the development of RBD protein as a viral attachment inhibitor and vaccine.Cellular & Molecular Immunology (2020) 17:613 – 620.
[3]Markus Hoffmann et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and is blocked by a Clinically Proven Protease Inhibitor. Cell 181, 271–280.
[4]Sana O.Alturki, et al. Current SARS-CoV-2 Vaccine Development.Frontiers in immunology, August 2020, Volume 11, Article 1880.