Helicobacter Pylori
Helicobacter Pylori (HP) is a type of bacteria that parasitizes the surface of the human gastric mucosa. Histologically, almost all HP infections show an active inflammatory response. This infectious disease can lead to chronic gastritis, peptic ulcers, and other related conditions. Common symptoms include upper stomach discomfort, pain, gas, anorexia, nausea, vomiting, and dark or tarry stools. Approximately 70% of infected individuals do not exhibit obvious symptoms. HP is the primary pathogenic factor for gastritis and peptic ulcers. It is closely associated with functional dyspepsia, gastric mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric cancer. The World Health Organization's International Agency for Research on Cancer and the United States have classified it as a definite Class I carcinogen.
Helicobacter pylori urease (HP-Ure) accounts for 6%-10% of the secreted proteins synthesized by Helicobacter pylori. Detecting HP-Ure can be used to determine if a patient is infected. Combined with CagA and VacA typing tests, as well as gastroscopy and pathological analysis, the degree of infection can be graded and management decisions can be made, including whether patients require eradication treatment based on their HP pathogenicity and clinical manifestations. Therefore, HP-Ure detection can help prevent or alleviate related diseases and enable early detection, diagnosis, and treatment of HP infection, which is clinically significant in guiding the treatment of gastrointestinal diseases.
HP has an infection rate of over 50% in natural populations worldwide. It is the only microorganism capable of colonizing the human stomach and is the primary pathogenic factor for peptic ulcers and chronic gastritis in humans. According to the World Health Organization's report, half of gastric cancer cases are associated with HP. Studies have confirmed that H. pylori infection is associated with a 6-fold higher risk of non-cardia gastric cancer and a 3-fold higher risk of cardia gastric cancer.
Currently, the main detection methods include invasive methods (gastric mucosal tissue section staining with a microscope, bacterial culture, rapid urease test) and non-invasive methods (13C or 14C urea breath test, fecal antigen detection, serum antibody detection). The most widely used method is the 13C or 14C urea breath test, but this test can only detect HP infection in the stomach and not outside of it. It is also not suitable for examination when the breath test results are near the critical value and cannot be judged.
Helicobacter Pylori (HP) recombinant proteins
Product Name | Cat. No. | Note | Application |
Recombinant H.pylori Cag A antigen | S00-CAGA1 | Antigen | LFA, ELISA |
Recombinant H.pylori Vac A antigen | S00-VACA1 | Antigen | LFA, ELISA |
Recombinant H.pylori UreA antigen | S00-UREA1 | Antigen | LFA, ELISA |
Recombinant H.pylori UreB antigen | S00-UREB1 | Antigen | LFA, ELISA |
Helicobacter Pylori (HP) is a type of bacteria that parasitizes the surface of the human gastric mucosa. Histologically, almost all HP infections show an active inflammatory response. This infectious disease can lead to chronic gastritis, peptic ulcers, and other related conditions. Common symptoms include upper stomach discomfort, pain, gas, anorexia, nausea, vomiting, and dark or tarry stools. Approximately 70% of infected individuals do not exhibit obvious symptoms. HP is the primary pathogenic factor for gastritis and peptic ulcers. It is closely associated with functional dyspepsia, gastric mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric cancer. The World Health Organization's International Agency for Research on Cancer and the United States have classified it as a definite Class I carcinogen.
Helicobacter pylori urease (HP-Ure) accounts for 6%-10% of the secreted proteins synthesized by Helicobacter pylori. Detecting HP-Ure can be used to determine if a patient is infected. Combined with CagA and VacA typing tests, as well as gastroscopy and pathological analysis, the degree of infection can be graded and management decisions can be made, including whether patients require eradication treatment based on their HP pathogenicity and clinical manifestations. Therefore, HP-Ure detection can help prevent or alleviate related diseases and enable early detection, diagnosis, and treatment of HP infection, which is clinically significant in guiding the treatment of gastrointestinal diseases.
HP has an infection rate of over 50% in natural populations worldwide. It is the only microorganism capable of colonizing the human stomach and is the primary pathogenic factor for peptic ulcers and chronic gastritis in humans. According to the World Health Organization's report, half of gastric cancer cases are associated with HP. Studies have confirmed that H. pylori infection is associated with a 6-fold higher risk of non-cardia gastric cancer and a 3-fold higher risk of cardia gastric cancer.
Currently, the main detection methods include invasive methods (gastric mucosal tissue section staining with a microscope, bacterial culture, rapid urease test) and non-invasive methods (13C or 14C urea breath test, fecal antigen detection, serum antibody detection). The most widely used method is the 13C or 14C urea breath test, but this test can only detect HP infection in the stomach and not outside of it. It is also not suitable for examination when the breath test results are near the critical value and cannot be judged.
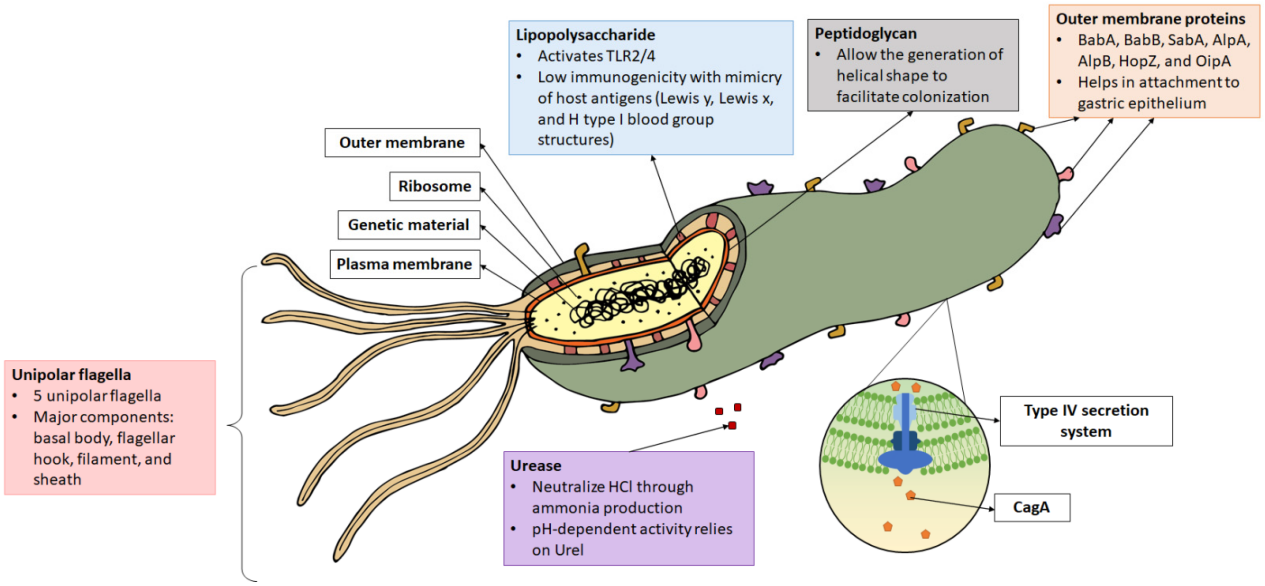
Recombinant H.pylori CagA antigen (34 kDa) [His]
CagA (cytotoxin-associated gene A), a virulence factor of Helicobacter pylori, is a protein with a molecular weight of 120-140kDa. It is encoded on the 40kb cag pathogenicity island (PAI). At the carboxyl end of CagA, there is a sequence called EPIYA, which consists of five repeating amino acids. The number and type of EPIYA sequences determine the biological activity of CagA.
When H. pylori translocates into gastric epithelial cells, the EPIYA sequence undergoes phosphorylation mediated by host kinases. This phosphorylation triggers a cascade of cell signaling events that impact intercellular adhesion, cell proliferation, IL-8 expression, and cell elongation. CagA has long been regarded as the most potent virulence factor of H. pylori.
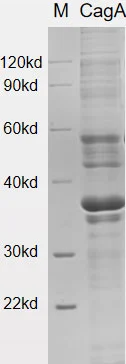
Recombinant H. pylori VacA Protein (30kDa) [His]
VacA (Vacuolating cytotoxin A) is a toxin produced by H. pylori that plays a role in generating vacuoles within host cells and inducing apoptosis. The VacA gene encodes a protein with a mass of approximately 140 kDa, but the mature VacA protein is around 88 kDa. Upon processing, VacA can be hydrolyzed into two fragments known as p33 and p55.
The VacA protein has various detrimental effects on gastric epithelial cells and the gastric mucosa. It can disrupt the cytoskeleton of gastric epithelial cells and cause damage to the gastric mucosa. This disruption provides favorable conditions for the survival and colonization of H. pylori. Additionally, VacA has the ability to translocate to mitochondria, leading to the release of cytochrome c and activation of pro-apoptotic factors, thereby contributing to apoptotic processes.
Furthermore, VacA is implicated in inducing inflammation in the stomach and promoting gastric cancer by interfering with autophagy responses. This disruption of autophagy mechanisms can contribute to the development and progression of gastric cancer.
In summary, VacA is a potent toxin produced by H. pylori that can induce vacuole formation, apoptosis, disruption of the cytoskeleton, and inflammation. Its actions play a significant role in the pathogenesis of H. pylori-related diseases, including gastric ulcers and gastric cancer.
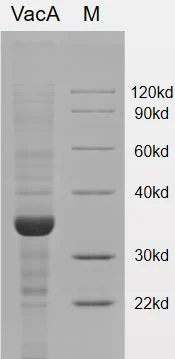
Recombinant H. pylori UreaA(26.6kDa) [His]
Recombinant H. pylori UreaB(61.44kDa) [His]
The urease enzyme of H. pylori consists of two subunits, namely the α subunit with a molecular weight of 61-66kDa and the β subunit with a molecular weight of 26-31kDa. These subunits combine to form a hexameric structure.
The primary function of urease is to catalyze the hydrolysis of urea, resulting in the production of ammonia. This ammonia production creates an alkaline environment that facilitates H. pylori colonization in the gastric mucosa. Additionally, urease can directly exert toxic effects on the gastric mucosa, leading to the formation of vacuoles.
Moreover, urease can activate mononuclear phagocytes and stimulate the production of inflammatory cytokines, such as tumor necrosis factor TNF-α and interleukin IL-6. This activation of immune cells and the subsequent release of inflammatory cytokines contribute to the inflammatory response associated with H. pylori infection.


















