Monkeypox Diagnosis Methods
Monkeypox virus (MPXV), smallpox and vaccinia all belong to the Orthopoxvirus genus. Monkeypox virus is mainly transmitted to humans by rodents, primates and other wild animals, and has limited human-to-human transmission, causing a rare viral zoonotic disease-monkeypox. On September 1st , 1970, the first human cases of MPXV were identified at Basankusu Hospital in Congo. Monkeypox virus is mainly transmitted to humans by rodents, primates and other wild animals, and has limited human-to-human transmission, causing a rare viral zoonotic disease-monkeypox. The clinical symptoms of monkeypox are similar to those of smallpox, but the symptoms are milder. Monkeypox mainly occurred in remote areas of Central and West Africa, where is near to tropical rainforests. With the adaptation to humans, MPXV gradually evolved into two clades: Central Africa branch (Congo Basin) and West Africa branch. Among them, the Congo Basin branch has stronger virulence and transmissibility.
Introduction to MPXV
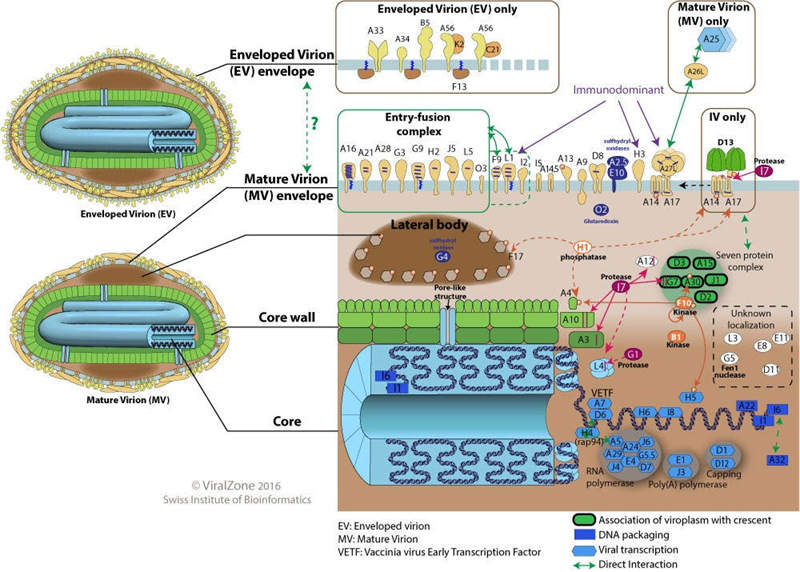
Monkeypox virus is an enveloped double-stranded DNA virus, that is very dependent on host cell DNA and RNA replication, and can replicate in the host cytoplasm. Similar to viruses of the same genus, MPXV is very likely to replicate the morphologically distinct intracellular mature virus (IMV) and extracellular enveloped virus (EEV) in the host cytoplasm. IMVs have a robust physical structure that facilitates transmission between hosts, while the more fragile EEVs are enveloped and limit immune clearance by the host. Therefore, EEVs are more suitable for virus-to-cell transmission. MPXV can effectively infect human primary monocytes, eliminating local T cell responses by inhibiting CD4+ and CD8+ T cell activation, so that avoiding systemic immunosuppression and immune monitoring.
Clinical diagnosis of monkeypox virus
The best diagnostic samples for monkeypox come from skin lesions: fluid from vesicles and pustules, and dry crusts. Lesion samples must be stored in dry, sterile tubes (like VTM) and kept cold. The clinical diagnosis of monkeypox must consider other rash disorders such as chickenpox, measles, bacterial skin infections, scabies, syphilis, and drug-related allergies. Lymphadenopathy in the early stages of disease may be the only clinical feature that distinguishes monkeypox from chickenpox or smallpox.
1. Electron microscope biopsy
Under the electron microscope, MPXV was brick-shaped in cytoplasm, with lateral bodies, and the central nucleus was about 200-300 nm long. Since OPV characterizations are morphologically indistinguishable, this method cannot confirm the diagnosis, but can suggest that the virus belongs to the Poxviridae genus.
2. Gene detection
2.1. Real-time fluorescence quantitative PCR
Routine detection of MPXV DNA on clinical samples and MPXV-infected cell cultures, with PCR or real-time PCR method. The detection is recommended to perform in a biosafety level 3 facility. Real-time PCR method should be used to detect the outer envelope protein gene (B6R), DNA polymerase gene, the conserved region of E9L, DNA-dependent RNA polymerase subunit 18, rpo18 and F3L gene.
2.2. Restriction length fragment polymorphism (RFLP)
PCR or restriction length fragment polymorphism (RELP) can also be used for the detection of MPXV DNA, but RFLP is time-consuming and requires virus culture. RFLP of PCR products also requires digestion followed by gel electrophoresis, which may not be the best approach in clinical settings where speed, sensitivity, and specificity are critical.
2.3. Next-generation sequencing technology (NGS)
Whole-genome sequencing with NGS method remains the gold standard for differentiating MPXV and other orthopoxvirus (OPV) characterization, but the technology is costly and downstream processing of sequencing data requires enormous computational power. Therefore, NGS may not be an appropriate approach in resource-limited countries in sub-Saharan Africa. Real-time PCR is still the preferred method for routine diagnosis of MPXV in genetic testing technology, but it must be supplemented by on-site genome sequencing technology. Such as Oxford Nanopore MinION, it provides real-time viral genome data, which is essential for evidence-based epidemiological intervention.
3. Immuno assay
Monkeypox virus immune assay mainly include enzyme-linked immunosorbent assay (ELISA) to detect IgG/IgM antibodies and immunohistochemical detection of viral antigens. Immunochemical analysis of MPXV antibodies can distinguish it from herpes virus. Antiviral antibodies and T-cell responses have been victimized to increase while the disease onset, with monkeypox virus IgM and IgG detected in serum around 5 days after the onset of the rash and that after more than 8 days. Possible MPXV infection should be diagnosed if IgM and IgG antibodies are present in the serum of unvaccinated individuals with a history of rash and severe illness. A positive IgM ELISA test indicates recent exposure to MPXV, while a positive IgG test indicates that the individual has been previously exposed to MPXV through vaccination or natural infection. The presence of both IgM and IgG in one sample indicates prior vaccination or exposure to individuals naturally infected with MPXV. However, orthopoxviruses are serologically cross-reactive, and antigen and antibody assays do not provide confirmation of monkeypox specificity, but may be feasible for serological testing in MPXV-endemic areas.
Reference:
[1] Sarah Keasey, Christine Pugh, Alexander Tikhonov, et al. Proteomic Basis of the Antibody Response to Monkeypox Virus Infection Examined in Cynomolgus Macaques and a Comparison to Human Smallpox Vaccination[J]. Plos one, 2010, 5(12): e15547.
[2] Emmanuel Alakunle, Ugo Moens, Godwin Nchind, et al.Monkeypox Virus in Nigeria: Infection Biology,Epidemiology, and Evolution[J]. Viruses, 2020, 12(11):1257.