Covid-19 LFA Uncut Sheet
Clinical Validation Result
Validated Product: COVID-19 antigen test (LFA) from SEKBIO Co., LTD.
Benchmark product: COVID-19 RT-PCR
Experiment 1: (Oropharyngeal swab eluted with matched sample extraction solution)
Number of samples | RT-PCR Results | SEKBIO COVID-19 antigen test result | ||
| CT values | Result | Result | Consistency | |
| 57 | ≤30 | 57 positives | 57 positives | 100% |
| 82 | ≤33 | 82 positives | 82 positives | 100% |
| 91 | ≤34 | 91 positives | 90 positives | 98.90% |
| 149 | ≤36 | 149 positives | 136 positives | 91.28% |
| 45 | ≥40 | 45 negatives | 44 negatives | 97.78% |
Experiment 2 : (Nasopharyngeal swab/Oropharyngeal swab kept in UTM)
| Number of samples | RT-PCR Results | SEKBIO COVID-19 antigen test result | ||
| CT values | Result | Result | Consistency | |
| 52 | ≤25.1 | 52 positives | 52 positives | 100% |
| 82 | ≤34.8 | 82 positives | 77 positives | 93.9% |
| 45 | >40 | 45 negatives | 45 negatives | 100% |
SARS-CoV-2 Antigen Rapid Test Strip Result
Performance Comparison Analysis SARS-CoV-2 Rapid Ag Test Uncut Sheet

Our Sars-Cov-2 antigen test can detect as low as 10 pg/ml
Test Report of the New Variants
1. Experiment purpose: The COVID-19 has produced a series of mutations during the pandemic. COVID-19 detection reagent (lateral flow chromatography) is now used to detect nucleocapsid protein (NP) recombinant proteins of variants from the UK, South Africa, the United States and Brazil, to verify the performance of the LFA assay on the variants.
2. Experiment materials
2.1. COVID-19 NP assay reagent (LFA), Batch No. 2101K402, the test results of NP recombinant protein was positive at 31.25pg/ mL and its result was weak positive at 20pg/mL.
2.2. NP recombinant protein of UK variant B.1.1.7, including mutation: D3L, S235F, expressed in HEK293 cell.
2.3. NP recombinant protein of South African variant B.1.351, including mutation: R203K, G204R, expressed in HEK293 cell.
2.4. NP recombinant protein of US variant B.1.2, including mutation: P67S, P199L, expressed in HEK293 cell.
2.5. NP recombinant protein of U Brazil variant B.1.1.28, including mutation: P80R, S235F, expressed in HEK293 cell.
3. Experiment method
3.1. The NP recombinant proteins of the four variants were diluted with sample diluent. The dilution ratio is as follows: 1/1K, 1/200K, 1/400K, 1/800K, 1/1600K, 1/3200K, 1/6400K, 1/12800K, 1/25800K
3.2. Use COVID-19 assay reagent (LFA) to detect the above diluted specimens. Each specimen is tested twice.
3.3. Add samples and interpret the results according to the operation methods in the instructions.
3.4. Record the results.
4. Experiment result
The experimental record results are shown as follows
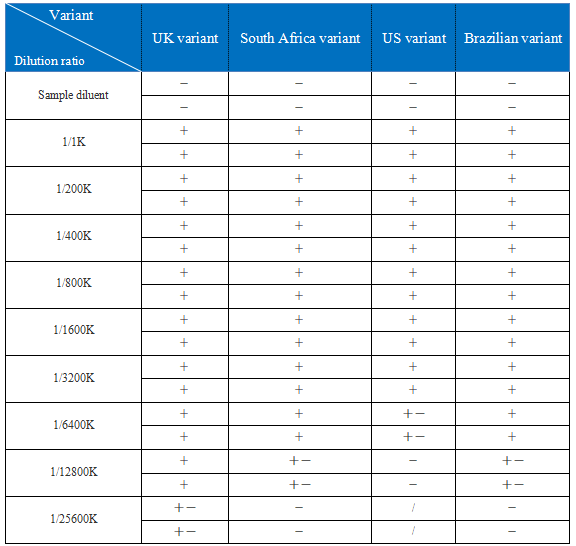
From the above table, the test result of NP recomb. protein of the UK variant was positive at dilution of 1/12800K and while it was slightly positive at dilution of 1/25600K. The test result of NP recombinant protein of the South African variant was positive at 1/6400K dilution and slightly positive at 1/12800K dilution, while it was negative at 1/25600K. The test result of NP recombinant protein of the American variant was positive at 1/3200K dilution and slightly positive at 1/6400K dilution, while it was negative at 1/12800K. The test result of NP recombinant protein of the Brazilian variant was positive at dilution of 1/6400K and slightly positive at dilution of 1/12800K, while it was negative at 1/25600K.
5. Experiment conclusion
The NP recombinant protein of the variant from the United Kingdom, South Africa, the United States and Brazil was detectable by the COVID-19 assay (LFA).
The NP recombinant protein of UK variant is detectable at the dilution ratio of 1/12800K.
The NP recombinant protein of US variant is detectable at the dilution ratio of 1/3200K.
The NP recombinant protein of Brazilian variant is detectable at the dilution ratio of 1/6400K.
The NP recombinant protein of South Africa variant is detectable at the dilution ratio of 1/6400K.

Analysis of the potential Microbial cross-reaction
1 Experimental purpose
To investigate the impact of the test result of the novel coronavirus (SARS-CoV-2) antigen detection kit (colloidal gold immunochromatography) when coexisting other microorganisms in the sample.
2 Experimental materials
2.1 Novel coronavirus (SARS-CoV-2) antigen detection kit (colloidal gold immunochromatography)
2.2 Microbial cross-reaction samples (Detail in Table 1)
2.3 SARS-CoV-2 virus culture
Table 1
| Sources | Sample No. | Sample Type |
Chinese "National controls for SARS-CoV-2 antigen detection kit" N1~N20 | N1 | Staphylococcus aureus |
| N2 | Streptococcus pyogenes | |
| N3 | Measles virus | |
| N4 | Paramyxovirus parotitis | |
| N5 | Adenovirus 3 | |
| N6 | Mycoplasma pneumoniae | |
| N7 | Parainfluenza virus 2 | |
| N8 | Human Metapneumovirus (hMPV) | |
| N9 | Human coronavirus OC43 | |
| N10 | Human coronavirus 229E | |
| N11 | Bordetella parapertussia | |
| N12 | Influenza B (Victoria strain) | |
| N13 | Influenza B (Ystrain) | |
| N14 | Influenza A (H1N1, 2009) | |
| N15 | Influenza A (H3N2) | |
| N16 | Avian influenza virus (H7N9) | |
| N17 | Avian influenza virus (H5N1) | |
| N18 | Epstein-Barr virus | |
| N19 | Enterovirus CA16 | |
| N20 | Rhinovirus | |
Zhujiang Hospital of Southern Medical University | N21 | Respiratory syncytial virus |
| N22 | Streptococcus pneumoniae | |
| N23 | Candida albicans | |
| N24 | Chlamydia pneumoniae | |
| N25 | Bordetella pertussis | |
| N26 | Human coronavirus NL63 (Recombinant protein) | |
| N27 | Pneumocystis jirovecii | |
| N28 | Mycobacterium tuberculosis | |
| N29 | Human coronavirus HKU1 (Recombinant protein) | |
| N30 | Legionella pneumophila |
3 Experimental method
Add the SARS-Cov-2 virus culture to the specimens in 2.2, to make the concentration of the virus culture three times of the minimum detection limit (1.5×102 TCID50/mL). Use the prepared specimens as samples to test in accordance with the instructions. 3 repeats per specimen, record the results and analyze the data. The sample numbers are as follows:
Table 2 Added sample and its No.
Sources | Sample No. | Added sample | Sample type |
Chinese "National controls for SARS-CoV-2 antigen detection kit" N1~N20 | N1 | N1+ | Staphylococcus aureus |
N2 | N2+ | Streptococcus pyogenes | |
N3 | N3+ | Measles virus | |
N4 | N4+ | Paramyxovirus parotitis | |
N5 | N5+ | Adenovirus 3 | |
N6 | N6+ | Mycoplasma pneumoniae | |
N7 | N7+ | Parainfluenza virus 2 | |
N8 | N8+ | Human Metapneumovirus (hMPV) | |
N9 | N9+ | Human coronavirus OC43 | |
N10 | N10+ | Human coronavirus 229E | |
N11 | N11+ | Bordetella parapertussia | |
N12 | N12+ | Influenza B (Victoria strain) | |
N13 | N13+ | Influenza B (Ystrain) | |
N14 | N14+ | Influenza A (H1N1, 2009) | |
N15 | N15+ | Influenza A (H3N2) | |
N16 | N16+ | Avian influenza virus (H7N9) | |
N17 | N17+ | Avian influenza virus (H5N1) |
N18 | N18+ | Epstein-Barr virus | |
N19 | N19+ | Enterovirus CA16 | |
N20 | N20+ | Rhinovirus | |
Zhujiang Hospital of Southern Medical University | N21 | N21+ | Respiratory syncytial virus |
N22 | N22+ | Streptococcus pneumoniae | |
N23 | N23+ | Candida albicans | |
N24 | N24+ | Chlamydia pneumoniae | |
N25 | N25+ | Bordetella pertussis | |
N26 | N26+ | Human coronavirus NL63(Recombinant protein) | |
N27 | N27+ | Pneumocystis jirovecii | |
N28 | N28+ | Mycobacterium tuberculosis | |
N29 | N29+ | Human coronavirus HKU1( Recombinant protein) | |
N30 | N30+ | Legionella pneumophila |
4 Experimental results
Table 3 Test results of interference reaction
Original Sample | Result | Added Sample | Result | ||||
Batch 1 | Batch 2 | Batch 3 | Batch 1 | Batch 2 | Batch 3 | ||
N1 | - | - | - | N1+ | + | + | + |
N2 | - | - | - | N2+ | + | + | + |
N3 | - | - | - | N3+ | + | + | + |
N4 | - | - | - | N4+ | + | + | + |
N5 | - | - | - | N5+ | + | + | + |
N6 | - | - | - | N6+ | + | + | + |
N7 | - | - | - | N7+ | + | + | + |
N8 | - | - | - | N8+ | + | + | + |
N9 | - | - | - | N9+ | + | + | + |
N10 | - | - | - | N10+ | + | + | + |
N11 | - | - | - | N11+ | + | + | + |
N12 | - | - | - | N12+ | + | + | + |
N13 | - | - | - | N13+ | + | + | + |
N14 | - | - | - | N14+ | + | + | + |
N15 | - | - | - | N15+ | + | + | + |
N16 | - | - | - | N16+ | + | + | + |
N17 | - | - | - | N17+ | + | + | + |
N18 | - | - | - | N18+ | + | + | + |
N19 | - | - | - | N19+ | + | + | + |
N20 | - | - | - | N20+ | + | + | + |
N21 | - | - | - | N21+ | + | + | + |
N22 | - | - | - | N22+ | + | + | + |
N23 | - | - | - | N23+ | + | + | + |
N24 | - | - | - | N24+ | + | + | + |
N25 | - | - | - | N25+ | + | + | + |
N26 | - | - | - | N26+ | + | + | + |
N27 | - | - | - | N27+ | + | + | + |
N28 | - | - | - | N28+ | + | + | + |
N29 | - | - | - | N29+ | + | + | + |
N30 | - | - | - | N30+ | + | + | + |
Note: "+" indicates a positive result, and "-" indicates a negative result.
5 Experimental conclusions
Cross-reaction experiments were conducted on three batches of reagents, and based on the results, the company’s novel coronavirus (SARS-CoV-2) antigen detection kit (colloidal gold immunochromatography) was used to detect the novel coronavirus (SARS-CoV-2). There are no false negatives and false positives for the specimens coexisting with other microorganisms. Therefore, the above microorganisms have no impact on the experimental results.
Uncut sheet of COVID-19 antigen detection
Product Name | Uncut sheet of COVID-19 antigen detection Novel coronavirus (SARS-CoV-2) antigen detection kit (colloidal gold immunochromatography)- detection membrane | |||
Spec. | 60mm*300mm | Lot No. | 2011K401 | |
Production quantity | 612 pieces | Sampling Amount | 8 pieces | |
Production Date | 2020-11-01 | Exp. | 2023-04 | |
Store at | sealed at 2⁓30℃ | Reported | 2020-11-02 | |
Inspection record | ||||
Test items | Acceptance Criteria | Result | Conclusion | |
Appearance | Clean, no scratches, smooth surface, no damage, no burrs, no stains; Each support of the large board should be firmly pasted. | ☑ Meet the requirements □ non-compliant | ☑ qualified □ Unqualified | |
Length (mm) | 300mm±3mm | 300mm | ☑ qualified □ Unqualified | |
C line, T line position | T line,B position should be 30mm±0.5mm; C line B position should be 34.5mm±0.5mm | T line, B position is:30.0mm C line, B position is:34.5mm | ☑ qualified □ Unqualified | |
Liquid moving speed (mm/min) | ≥10mm/min | 57.5mm/min | ☑ qualified □ Unqualified | |
Fading time of film (min) | 15~20min | 16min | ☑ qualified □ Unqualified | |
Positive rate of positive reference | Use the corporate positive reference product P1-P5 to test, The result should meet the requirement as follows: The color intensity of P1 should be C8-C7; | ☑ Meet the requirements □ non-compliant | ☑ qualified □ Unqualified | |
The color intensity of P2 should be C6~C5; The color intensity of P3 should be C5~C4; The color intensity of P4 should be C8~C7; The color intensity of P5 should be C7~C6. | ||||
Minimum detection limit | Use corporate lowest detection limit reference product L1⁓L3 for testing, the result should meet the requirement as follows: The color intensity of L1 should be B; The color intensity of L2 should be C9~C7; The color intensity of L3 should be C8~C7. | ☑ Meet the requirements □ non-compliant | ☑ qualified □ Unqualified | |
Precision | Use corporate repeatable reference product R1⁓R2 to test, and the result should meet the requirement as follows: The color intensity of R1 should be C6~C5; The color intensity of R2 should be C7~C6. | ☑ Meet the requirements □ non-compliant | ☑ qualified □ Unqualified | |
Negative rate of Negative reference | Test corporate negative reference product N1⁓N7, all color intensity should be B. | ☑ Meet the requirements □ non-compliant | ☑ qualified □ Unqualified | |
Random Clinical Sample | Sampling 100 samples randomly, using the sample extraction solution to elute the sample and test, The test color intensity should be B~C9. Sampling 20 samples randomly in UTM tube with COPAN preserved solution, and test, The test color intensity should be B~C9. | The number of color intensity of B~C9/total number of tests: 1 20 / 120 | ☑ qualified □ Unqualified | |
The total false positive rate should be ≤2% | Total false positive rate: 0 | ☑ qualified □ Unqualified | ||
Remark | / | |||
Conclusion: | ☑ qualified □ Unqualified | |||














